Dimethylheptylpyran
 |
|
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 20–39 hours |
| Identifiers | |
|
|
| CAS Number |
32904-22-6 |
| PubChem (CID) | 36276 |
| ChemSpider |
33359 |
| UNII |
944O1KA97G |
| ChEMBL |
CHEMBL3244434 |
| Chemical and physical data | |
| Formula | C25H38O2 |
| Molar mass | 370.57 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
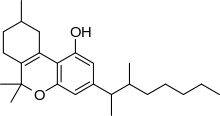
Dimethylheptylpyran (DMHP, 3-(1,2-dimethylheptyl)-Δ6a,10a-THC, 1,2-dimethylheptyl-Δ3THC, A-40824, EA-2233) is a synthetic analogue of THC, which was invented in 1949 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. DMHP is a pale yellow, viscous oil which is insoluble in water, but dissolves in alcohol or non-polar solvents.
DMHP is similar in structure to THC, differing only in the position of one double bond, and the replacement of the 3-pentyl chain with a 3-(1,2-dimethylheptyl) chain. It produces similar activity to THC, such as sedative effects, but is considerably more potent, especially having much stronger analgesic and anticonvulsant effects than THC, although comparatively weaker psychological effects. It is thought to act as a CB1 agonist, in a similar manner to other cannabinoid derivatives.
DMHP and its O-acetate ester were extensively investigated by the US military chemical weapons program in the Edgewood Arsenal experiments, as possible non-lethal incapacitating agents.
DMHP has three stereocenters and consequently has eight possible stereoisomers, which differ considerably in potency. The racemic mix of all eight isomers of the O-acetyl ester was given the code number EA-2233, with the eight individual isomers numbered EA-2233-1 through EA-2233-8. The most potent isomer was EA-2233-2, with an active dose range in humans of 0.5–2.8 μg/kg (i.e. ~35–200 μg for a 70 kg adult). Active doses varied markedly between individuals, but when the dose of EA-2233 was taken up to 1–2 mg, all volunteers were considered to be incapable of performing military duties, with the effects lasting as long as 2–3 days.
...
Wikipedia
