Dimercaprol
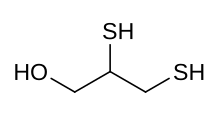 |
|

Skeletal formula and ball and stick model of dimercaprol
|
|
| Clinical data | |
|---|---|
| Trade names | BAL in Oil |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
intramuscular |
| ATC code | |
| Identifiers | |
| Synonyms | 2,3-Dimercaptopropanol British anti-Lewisite 2,3-Dimercaptopropan-1-ol (no longer recommended) British antilewisite |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.000.394 |
| Chemical and physical data | |
| Formula | C3H8OS2 |
| Molar mass | 124.225 g mol−1 |
| 3D model (Jmol) | |
| Density | 1.239 g cm−3 g/cm3 |
| Boiling point | 393 °C (739 °F) at 2.0 kPa |
|
|
|
|
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. May also be used for antimony, thallium, or bismuth poisoning but the evidence is not good for these uses. It is given by injection into a muscle.
Common side effects include high blood pressure, pain at the site of injection, vomiting, and fever. It is not recommended in people with peanut allergies. It is unclear if use in pregnancy is safe for the baby. Dimercaprol works by binding with heavy metals.
Dimercaprol was first made during World War II. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United States a course of treatment costs more than 200 USD.
Dimercaprol has long been the mainstay of chelation therapy for lead or arsenic poisoning, and it remains an essential drug. Nonetheless, because it can have serious adverse effects, researchers have also pursued development of less toxic analogues.
Arsenic and some other heavy metals act by chemically reacting with adjacent thiol residues on metabolic enzymes, creating a chelate complex that inhibits the affected enzyme's activity. Dimercaprol competes with the thiol groups for binding the metal ion, which is then excreted in the urine.
...
Wikipedia
