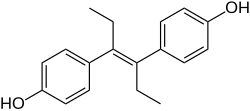
Diethylstilbestrol
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration |
Oral, intravenous |
| ATC code | G03CB02 (WHO) G03CC05 (WHO), L02AA01 (WHO) |
| Identifiers | |
|
|
| CAS Number |
56-53-1 |
| PubChem (CID) | 448537 |
| IUPHAR/BPS | 2801 |
| DrugBank |
DB00255 |
| ChemSpider |
395306 |
| UNII |
731DCA35BT |
| KEGG |
D00577 |
| ChEBI |
CHEBI:41922 |
| ChEMBL |
CHEMBL411 |
| ECHA InfoCard | 100.000.253 |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.35 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Diethylstilbestrol (DES) (INN, USAN, BAN), also known formerly (and inappropriately) as stilboestrol (BAN), is a synthetic, non-steroidal estrogen of the stilbestrol group that was first synthesized in 1938. It is also classified as an endocrine disruptor. Human exposure to DES occurred through diverse sources, such as dietary ingestion from supplemented cattle feed and medical treatment for certain conditions, including breast and prostate cancers. From about 1940 to 1971, DES was given to pregnant women in the mistaken belief it would reduce the risk of pregnancy complications and losses.
In 1971, DES was shown to cause clear cell carcinoma, a rare vaginal tumor in girls and women who had been exposed to this drug in utero. The United States Food and Drug Administration subsequently withdrew DES from use in pregnant women. Follow-up studies have indicated that DES also has the potential to cause a variety of significant adverse medical complications during the lifetimes of those exposed. The United States National Cancer Institute recommends women born to mothers who took DES undergo special medical exams on a regular basis to screen for complications as a result of the drug. Individuals who were exposed to DES during their mothers' pregnancies are commonly referred to as "DES daughters" and "DES sons".
DES has been used in the past for the following indications:
An estimated 3 million pregnant women in the USA were prescribed DES from 1941 through 1971. DES was also widely prescribed to women in Canada, the UK, Europe, Australia, and New Zealand during a similar period. Women who were prescribed DES during pregnancy have been shown to have a modestly increased risk of breast cancer and breast cancer mortality.
DES gained notoriety when it was shown to cause a rare vaginal tumor in girls and young women who had been exposed to this drug in utero. In 1971, the New England Journal of Medicine published a report showing that seven of eight girls and young women (ages 14 to 22) who had been diagnosed with vaginal clear cell adenocarcinoma had been exposed prenatally to DES. Subsequent studies have shown an approximate 40-fold increased risk of vaginal/cervical clear cell adenocarcinoma in women exposed in utero to DES. As a consequence of this evidence, DES is considered an established human carcinogen. DES was one of the first transplacental carcinogens discovered in humans, meaning a toxin could cross the placenta and harm the fetus. It had originally been believed that the placenta protected the developing fetus but it is now known that is not true. Daughters exposed to DES in utero may also have an increased risk of moderate to severe cervical squamous cell dysplasia and an increased risk of breast cancer.
...
Wikipedia
