Diethyl carbonate
 |
|
| Names | |
|---|---|
| Other names
carbonic ether; ethyl carbonate; Eufin
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.011 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C5H10O3 | |
| Molar mass | 118.13 g/mol |
| Appearance | Clear liquid |
| Density | 0.975 g/cm3 |
| Melting point | −74.3 °C (−101.7 °F; 198.8 K) |
| Boiling point | 126 to 128 °C (259 to 262 °F; 399 to 401 K) |
| Insoluble | |
| Hazards | |
|
EU classification (DSD) (outdated)
|
Flammable (F) |
| R-phrases (outdated) | R11 |
| S-phrases (outdated) | S9 S16 S29 S33 |
| Flash point | 33 °C (91 °F; 306 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
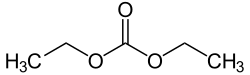
Diethyl carbonate is a carbonate ester of carbonic acid and ethanol with the formula OC(OCH2CH3)2. At room temperature (25 °C) diethyl carbonate is a clear liquid with a low flash point.
Diethyl carbonate is used as a solvent such as in erythromycin intramuscular injections. It can be used as a component of electrolytes in lithium batteries. It has been proposed as a fuel additive to support cleaner diesel fuel combustion because its high boiling point might reduce blended fuels' volatility, minimize vapor buildup in warm weather that can block fuel lines.
It can be made by reacting phosgene with ethanol, producing hydrogen chloride as a byproduct. Because chloroform can react with oxygen to form phosgene, chloroform is stabilized for storage by adding 1 part (by mass) of ethanol to 100 parts (by mass) of chloroform, so that any phosgene that forms is converted into diethyl carbonate.
...
Wikipedia
