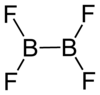
Diboron tetrafluoride
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Diboron tetrafluoride
|
|||
|
Systematic IUPAC name
Tetrafluorodiborane
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| B2F4 | |||
| Molar mass | 97.616 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 4.3 kg/m3 (gas) | ||
| Melting point | −56 °C (−69 °F; 217 K) | ||
| Boiling point | −34 °C (−29 °F; 239 K) | ||
| Thermochemistry | |||
| 79.1 J/mol K | |||
|
Std molar
entropy (S |
317.3 J/mol K | ||
|
Std enthalpy of
formation (ΔfH |
-1440.1 kJ/mol | ||
|
Gibbs free energy (ΔfG˚)
|
-1410.4 kJ/mol | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
Diboron tetrafluoride is a colorless gas. It can be formed by reacting boron monofluoride with boron trifluoride at low temperatures, taking care not to form higher polymers.
...
Wikipedia