Decane
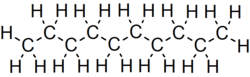 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Decane
|
|
| Identifiers | |
|
124-18-5 |
|
| 3D model (Jmol) | Interactive image |
| 1696981 | |
| ChEBI |
CHEBI:41808 |
| ChEMBL |
ChEMBL134537 |
| ChemSpider |
14840 |
| DrugBank |
DB02826 |
| ECHA InfoCard | 100.004.262 |
| EC Number | 204-686-4 |
| MeSH | decane |
| PubChem | 15600 |
| RTECS number | HD6550000 |
| UN number | 2247 |
|
|
|
|
| Properties | |
| C10H22 | |
| Molar mass | 142.29 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 0.730 g mL−1 |
| Melting point | −30.5 to −29.2 °C; −22.8 to −20.6 °F; 242.7 to 243.9 K |
| Boiling point | 173.8 to 174.4 °C; 344.7 to 345.8 °F; 446.9 to 447.5 K |
| log P | 5.802 |
| Vapor pressure | 195 Pa |
|
Henry's law
constant (kH) |
2.1 nmol Pa−1 kg−1 |
| -119.74·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.411–1.412 |
| Viscosity | 920 μPa s (at 20 °C) |
| Thermochemistry | |
| 315.46 J K−1 mol−1 | |
|
Std molar
entropy (S |
425.89 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−302.1–−299.9 kJ mol−1 |
|
Std enthalpy of
combustion (ΔcH |
−6779.21–−6777.45 kJ mol−1 |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms |
 
|
| GHS signal word | DANGER |
| H226, H304 | |
| P301+310, P331 | |
|
EU classification (DSD)
|
|
| R-phrases | R10, R65 |
| NFPA 704 | |
| Flash point | 46.0 °C (114.8 °F; 319.1 K) |
| 210.0 °C (410.0 °F; 483.1 K) | |
| Explosive limits | 0.8–2.6% |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
|
| Related compounds | |
|
Related alkanes
|
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Decane is an alkane hydrocarbon with the chemical formula C10H22. Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3. All isomers, however, exhibit similar properties and little attention is paid to the composition. These isomers are flammable liquids. Decane is a component of gasoline (petrol) and kerosene. Like other alkanes, it is nonpolar solvent, does not dissolve in water, and is readily combustable. Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes.
Decane undergoes combustion, just like other alkanes. In the presence of sufficient oxygen, it burns to form water and carbon dioxide.
With insufficient oxygen, carbon monoxide is also formed.
It has a surface tension of 0.0238 N·m−1.
...
Wikipedia

