Dacarbazine
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /dəˈkɑːrbəˌziːn/ |
| Trade names | DTIC-Dome, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682750 |
| Pregnancy category |
|
| Routes of administration |
IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | Extensive |
| Biological half-life | 5 hours |
| Excretion | Kidney (40% as unchanged dacarbazine) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.022.179 |
| Chemical and physical data | |
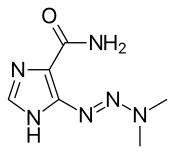
| Formula | C6H10N6O |
| Molar mass | 182.18 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Dacarbazine (DTIC), also known as imidazole carboxamide, is a chemotherapy medication used in the treatment of melanoma and Hodgkin's lymphoma. For Hodgkin's it is often used together with vinblastine, bleomycin, and doxorubicin. It is given by injection into a vein.
Common side effects include loss of appetite, vomiting, low white blood cell count, and low platelets. Other serious side effects include liver problems and allergic reactions. It is unclear if use in pregnancy is safe for the baby. Dacarbazine is in the alkylating agent and purine analog families of medication.
Dacarbazine was approved for medical use in the United States in 1975. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about 7.45 to 18.24 USD per 200 mg vial. In the United Kingdom this dose costs the NHS about 7.50 pounds.
As of mid-2006, dacarbazine is commonly used as a single agent in the treatment of metastatic melanoma, and as part of the ABVD chemotherapy regimen to treat Hodgkin's lymphoma, and in the MAID regimen for sarcoma. Dacarbazine was proven to be just as efficacious as procarbazine in the German trial for paediatric Hodgkin's lymphoma, without the teratogenic effects. Thus COPDAC has replaced the former COPP regime in children for TG2 & 3 following OEPA.
...
Wikipedia
