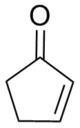
Cyclopentenone
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
2-Cyclopenten-1-one
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.012.012 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C5H6O | |||
| Molar mass | 82.04 g·mol−1 | ||
| Density | 0.98 g·mL−1 | ||
| Boiling point | 150 °C (302 °F; 423 K) | ||
| almost insoluble in water | |||
| Hazards | |||
| Main hazards | Harmful | ||
| Flash point | 42 °C (108 °F; 315 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2-Cyclopentenone is a hydrocarbon with chemical formula C5H6O and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. 2-Cyclopentenone contains two functional groups, a ketone and an alkene. It is a colorless liquid.
The term cyclopentenone may also refer to a structural motif wherein the cyclopentenone moiety is a subunit of a larger molecule. Cyclopentenones are found in a large number of natural products, including jasmone, the aflatoxins, and several prostaglandins.
2-Cyclopentenones can be synthesized in a number of ways. Another route involves elimination of α-bromo-cyclopentanone using lithium carbonate and Claisen condensation-decarboxylation-isomerization cascades of unsaturated diesters as shown below.
The acid-catalyzed dehydration of cyclopentanediols affords cyclopentenone.
As a functional group, the synthesis of 2-cyclopentenones is accomplished in a variety of other ways, including the Nazarov cyclization reaction from divinyl ketones, Saegusa–Ito oxidation from cyclopentanones, ring-closing metathesis from the corresponding dienes, oxidation of the corresponding cyclic allylic alcohols, and the Pauson–Khand reaction from alkenes, alkynes, and carbon monoxide.
...
Wikipedia