Contergan
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /θəˈlɪdəmaɪd/ |
| Trade names | Thalomid, Immunoprin, Talidex, Talizer, Neurosedyn (S) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth (capsules) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Protein binding | 55% and 66% for the (R)-(+)- and (S)-(−)-enantiomers, respectively |
| Metabolism | Liver (minimally via CYP2C19-mediated 5-hydroxylation; mostly via non-enzymatic hydrolysis at the four amide sites) |
| Biological half-life | 5–7.5 hours (dose-dependent) |
| Excretion | Urine, faeces |
| Identifiers | |
|
|
| Synonyms | α-(N-Phthalimido)glutarimide |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.029 |
| Chemical and physical data | |
| Formula | C13H10N2O4 |
| Molar mass | 258.23 g·mol−1 |
| 3D model (Jmol) | |
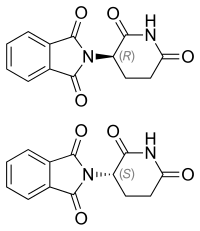
| Chirality | Racemic mixture |
|
|
|
|
|
|
|
Thalidomide, sold under the brand name Immunoprin, among others, is an immunomodulatory drug and the prototype of the thalidomide class of drugs. Today, thalidomide is used mainly as a treatment of certain cancers (multiple myeloma) and of a complication of leprosy. It can be readily synthesized to yield large quantities of the drug in just two steps.
Thalidomide was first marketed in 1957 in West Germany under the trade-name Contergan. The German drug company Chemie Grünenthal developed and sold the drug. Primarily prescribed as a sedative or hypnotic, thalidomide also claimed to cure "anxiety, insomnia, gastritis, and tension". Afterwards, it was used against nausea and to alleviate morning sickness in pregnant women. Thalidomide became an over-the-counter drug in West Germany on October 1, 1957. Shortly after the drug was sold in West Germany, between 5,000 and 7,000 infants were born with phocomelia (malformation of the limbs). Only 40% of these children survived. Throughout the world, about 10,000 cases were reported of infants with phocomelia due to thalidomide; only 50% of the 10,000 survived. Those subjected to thalidomide while in the womb experienced limb deficiencies in a way that the long limbs either were not developed or presented themselves as stumps. Other effects included deformed eyes and hearts, deformed alimentary and urinary tracts, blindness and deafness. The negative effects of thalidomide led to the development of more structured drug regulations and control over drug use and development.
Thalidomide is used for a number of conditions including erythema nodosum leprosum, multiple myeloma (in combination with dexamethasone), and various other cancers, for some symptoms of HIV/AIDS, Crohn's disease, sarcoidosis, graft-versus-host disease, rheumatoid arthritis, Behçet's disease and a number of skin conditions that have not responded to usual treatment.
...
Wikipedia
