Clobazam
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Frisium, Urbanol, Onfi, Tapclob |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral (tablets and Oral Suspension) |
| ATC code | N05BA09 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87% (oral) |
| Protein binding | 80–90% |
| Metabolism | Hepatic |
| Biological half-life | clobazam: 36–42 hours, N-desmethylclobazam: 71–82h |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number |
22316-47-8 |
| PubChem (CID) | 2789 |
| IUPHAR/BPS | 7149 |
| DrugBank |
DB00349 |
| ChemSpider |
2687 |
| UNII |
2MRO291B4U |
| KEGG |
D01253 |
| ChEBI |
CHEBI:31413 |
| ChEMBL |
CHEMBL70418 |
| ECHA InfoCard | 100.040.810 |
| Chemical and physical data | |
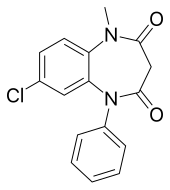
| Formula | C16H13ClN2O2 |
| Molar mass | 300.74 |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Clobazam (marketed under the brand names Frisium, Urbanol, Onfi and Tapclob ) is a benzodiazepine that has been marketed as an anxiolytic since 1975 and an anticonvulsant since 1984.
Clobazam is used for epilepsy. It is unclear if there are any benefits to clobazam over other seizure medications for children with Rolandic epilepsy or other epileptic syndromes.
As of 2005, clobazam is approved in Canada for add-on use in tonic-clonic, complex partial, and myoclonic seizures. Clobazam is approved for adjunctive therapy in complex partial seizures certain types of status epilepticus, specifically the myoclonic, myoclonic-absent, simple partial, complex partial, and tonic varieties, and non-status absence seizures. It is also approved for treatment of anxiety. In India, clobazam is approved for use as an adjunctive therapy in epilepsy and in acute and chronic anxiety. In Japan, clobazam is approved for adjunctive therapy in treatment-resistant epilepsy featuring complex partial seizures. In New Zealand, clobazam is marketed as Frisium In the United Kingdom clobazam (Frisium) is approved for short-term (2–4 weeks) relief of acute anxiety in patients who have not responded to other drugs, with or without insomnia and without uncontrolled clinical depression. It was not approved in the US until October 25, 2011, when it was approved for the adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome in patients 2 years of age or older.
It is also approved for adjunctive therapy for epilepsy in patients who have not responded to first-line drugs and in children who are refractory to first-line drugs. It is not recommended for use in children between the ages of six months and three years, unless there is a compelling need. In addition to epilepsy and severe anxiety, clobazam is also approved as a short-term (2–4 weeks) adjunctive agent in schizophrenia and other psychotic disorders to manage anxiety or agitation.
...
Wikipedia
