Cholestanol
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
5β-cholestan-3β-ol
|
|
| Other names
5β-coprostanol
coprostanol |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.036 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C27H48O | |
| Molar mass | 388.6756 g/mol |
| Melting point | 102 °C (216 °F; 375 K) |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
|
Related Stanols
|
24-ethyl coprostanol 5α-cholestanol epi-coprostanol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
5β-Coprostanol (5β-cholestan-3β-ol) is a 27-carbon stanol formed from the biohydrogenation of cholesterol (cholest-5en-3β-ol) in the gut of most higher animals and birds. This compound has frequently been used as a biomarker for the presence of human faecal matter in the environment.
5β-coprostanol has a low water solubility, and consequently a high octanol – water partition coefficient (log Kow = 8.82). This means that in most environmental systems, 5β-coprostanol will be associated with the solid phase.
In anaerobic sediments and soils, 5β-coprostanol is stable for many hundreds of years enabling it to be used as an indicator of past faecal discharges. As such, records of 5β-coprostanol from paleo-environmental archives have been used to further constrain the timing of human settlements in a region, as well as reconstruct relative changes in human populations and agricultural activities over several thousand years.
Since the molecule has a hydroxyl (-OH) group, it is frequently bound to other lipids including fatty acids; most analytical methods, therefore, utilise a strong alkali (KOH or NaOH) to saponify the ester linkages. Typical extraction solvents include 6% KOH in methanol. The free sterols and stanols (saturated sterols) are then separated from the polar lipids by partitioning into a less polar solvent (e.g. hexane). Prior to analysis, the hydroxyl group is frequently derivatised with BSTFA (bis-trimethyl silyl trifluoroacetamide) to replace the hydrogen with the less exchangeable trimethylsilyl (TMS) group. Instrumental analysis is frequently conducted on gas chromatograph (GC) with either a flame ionisation detector (FID) or mass spectrometer (MS). The mass spectrum for 5β-coprostanol - TMS ether can be seen in the figure.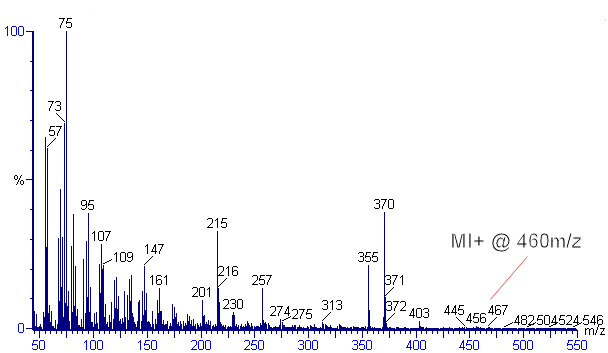
...
Wikipedia
