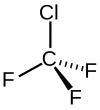
Chlorotrifluoromethane
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Chloro(trifluoro)methane
|
|||
| Other names
Chlorotrifluoromethane
Monochlorotrifluoromethane Trifluorochloromethane Trifluoromethyl chloride Trifluoromonochlorocarbon Arcton 3 Freon 13 Genetron 13 R-13 CFC 13 UN 1022 |
|||
| Identifiers | |||
|
75-72-9 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
6152 |
||
| ECHA InfoCard | 100.000.814 | ||
| EC Number | 200-894-4 | ||
| PubChem | 6392 | ||
| RTECS number | PA6410000 | ||
| UNII |
7C6U91JNED |
||
|
|||
|
|||
| Properties | |||
| CClF3 | |||
| Molar mass | 104.46 g/mol | ||
| Appearance | Colorless gas with sweet odor | ||
| Density | 1.526 g/cm3 | ||
| Melting point | −181 °C (−293.8 °F; 92.1 K) | ||
| Boiling point | −81.5 °C (−114.7 °F; 191.7 K) | ||
| 0.009% at 25 °C (77 °F) | |||
| Vapor pressure | 3.263 MPa at 21 °C (70 °F) | ||
| Hazards | |||
| Main hazards | Ozone depletor | ||
| Safety data sheet | ICSC 0420 | ||
| Flash point | Non-flammable | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive chlorofluorocarbon (CFC) and also a mixed halomethane. It is used as a refrigerant, however, due to concerns about its ozone-depleting potential, its use has been phased out due to the .
It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride:
CCl4 + 3HF → CClF3 + 3HCl
This reaction can also produce trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and tetrafluoromethane (CF4).
...
Wikipedia