Cetrorelix
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Cetrotide |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration |
Subcutaneous injection |
| ATC code | H01CC02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 86% |
| Biological half-life | 62.8 hours / 3mg single dose; 5 hours / 0.25 mg single dose; 20.6 hours / 0.25mg multiple doses |
| Excretion | feces (5% to 10% as unchanged drug and metabolites); urine (2% to 4% as unchanged drug) |
| Identifiers | |
|
|
| CAS Number |
120287-85-6 |
| PubChem (CID) | 16130924 |
| IUPHAR/BPS | 1190 |
| DrugBank |
DB00050 |
| ChemSpider |
10482082 |
| UNII |
OON1HFZ4BA |
| KEGG |
D07665 |
| ChEBI |
CHEBI:59224 |
| ChEMBL |
CHEMBL1200490 |
| ECHA InfoCard | 100.212.148 |
| Chemical and physical data | |
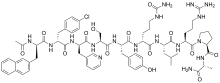
| Formula | C70H92ClN17O14 |
| Molar mass | 1431.06 g/mol |
|
|
|
|
|
Cetrorelix (INN, BAN), or cetrorelix acetate (USAN, JAN), is an injectable gonadotropin-releasing hormone (GnRH) antagonist that is marketed primarily under the brand name Cetrotide. A synthetic decapeptide, it is used is used in assisted reproduction to inhibit premature luteinizing hormone surges The drug works by blocking the action of GnRH upon the pituitary, thus rapidly suppressing the production and action of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, cetrorelix can be used to treat hormone-sensitive cancers of the prostate and breast (in pre-/perimenopausal women) and some benign gynaecological disorders (endometriosis, uterine fibroids and endometrial thinning). It is administered as either multiple 0.25 mg daily subcutaneous injections or as a single-dose 3 mg subcutaneous injection. The duration of the 3 mg single dose is four days; if hCG is not administered within four days, a daily 0.25 mg dose is started and continued until hCG is administered.
Cetrorelix is marketed by Merck Serono for use in in-vitro fertilization in all countries except Japan, where it is marketed by Shionogi and Nippon Kayaku. Aeterna Zentaris receives royalties on these sales and retains rights to develop cetrorelix for other indications. In IVF use it is injected daily after follicle stimulation has been initiated and evidence of follicle maturation is approaching; given daily it prevents an endogenous LH surge that would trigger an untimely ovulation prior to the hCG administration by the treating physician. As an alternative to the GnRH antagonist, also a GnRH agonist could be given, but agonist have to be started earlier to overcome the agonistic effect. Cetrorelix can be mixed with follitropin alpha without compromising their reported safety and efficacy.
...
Wikipedia
