Cefaclor
 |
|
| Clinical data | |
|---|---|
| Trade names | Biocef, Ceclor, Medacef,Distaclor, Keflor, Raniclor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682729 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed, independent of food intake |
| Metabolism | 15% to 40% |
| Biological half-life | 0.6 to 0.9 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.053.536 |
| Chemical and physical data | |
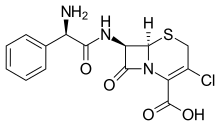
| Formula | C15H14ClN3O4S |
| Molar mass | 367.808 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Cefaclor, developed by Eli Lilly under the trade name Ceclor, is a second-generation cephalosporin antibiotic used to treat some infections caused by bacteria such as pneumonia and infections of the ear, lung, skin, throat, and urinary tract. It is also available from other manufacturers as a generic.
Cefaclor belongs to the family of antibiotics known as the cephalosporins (cefalosporins). The cephalosporins are broad-spectrum antibiotics that are used for the treatment of septicaemia, pneumonia, meningitis, biliary tract infections, peritonitis, and urinary tract infections. The pharmacology of the cephalosporins is similar to that of the penicillins, excretion being principally renal. Cephalosporins penetrate the cerebrospinal fluid poorly unless the meninges are inflamed; cefotaxime is a more suitable cephalosporin than cefaclor for infections of the central nervous system, e.g. meningitis. Cefaclor is active against many bacteria, including both Gram-negative and Gram-positive organisms.
Cautions include known sensitivity to beta-lactam antibacterials, such as penicillins (Cefaclor should be avoided if there is a history of immediate hypersensitivity reaction); renal impairment (no dose adjustment required, although manufacturer advises caution); pregnancy and breast-feeding (but appropriate to use); false positive urinary glucose (if tested for reducing substances) and false positive Coombs test. Cefaclor has also been reported to cause a serum sickness-like reaction in children.
...
Wikipedia
