Carboprost
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600042 |
| Pregnancy category |
|
| Routes of administration |
Intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
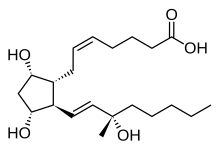
| Formula | C21H36O5 |
| Molar mass | 368.508 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Carboprost (INN, trade names for the tromethamine salts Hemabate, Tham) is a synthetic prostaglandin analogue of PGF2α (specifically, it is 15-methyl-PGF2α) with properties.
Carboprost induces contractions and can trigger abortion in early pregnancy. It also reduces postpartum bleeding.
Used in postpartum hemorrhage caused by uterine atony not controlled by other methods. One study has shown that carboprost tromethamine is more effective than oxytocin in preventing postpartum hemorrhage in high-risk patients undergoing caesarian delivery. Carboprost is also used for the termination of pregnancy in the 2nd trimester.
Unlabeled use:
Contraindicated in severe cardiovascular, renal, and hepatic disease. It is also contraindicated in acute Pelvic Inflammatory Disease. Hypersensitivity to carboprost or any of its components is also a contraindication Exert caution in asthmatic patients as carboprost may cause bronchospasm.
Carboprost is supplied with its salt derivative tromethamine in 1 milliliter ampules containing a 250 microgram/milliliter solution of the active drug. The drug must be refrigerated at a temperature between 2 – 8 degrees Celsius.
A significant deactivating metabolic transformation of natural prostaglandins is enzymatic oxidation of the C-15 hydroxyl to the corresponding ketone. This is prevented, with retention of activity, by methylation to give the C-15 tertiary carbinol series.
This molecular feature is readily introduced at the stage of the Corey lactone (1) by reaction with methyl Grignard reagent or trimethylaluminium. The resulting mixture of tertiary carbinols (2) is transformed to oxytocic carboprost (3) by standard transformations, including sepoaration of diastereomers, so that the final product is the C-15 analogue. This diastereomer is reputably freeer of porstaglandin side effects than the C-15 (S) isomer.
...
Wikipedia
