Calcium hypochlorite
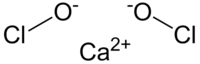 |
|
| Names | |
|---|---|
| Other names
Hypochlorous acid, calcium salt
Bleaching powder, Calcium oxychloride |
|
| Identifiers | |
|
7778-54-3 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
22912 |
| ECHA InfoCard | 100.029.007 |
| EC Number | 231-908-7 |
| PubChem | 24504 |
| RTECS number | NH3485000 |
| UN number | 1748 |
|
|
|
|
| Properties | |
| Ca(OCl)2 | |
| Molar mass | 142.98 g/mol |
| Appearance | white/gray powder |
| Density | 2.35 g/cm3 (20 °C) |
| Melting point | 100 °C (212 °F; 373 K) |
| Boiling point | 175 °C (347 °F; 448 K) decomposes |
| 21 g/100 mL, reacts | |
| Solubility | reacts in alcohol |
| Hazards | |
| Safety data sheet | ICSC 0638 |
|
EU classification (DSD)
|
|
| R-phrases | R8, R22, R31, R34, R50 |
| S-phrases | (S1/2), S26, S36/37/39, S45, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
850 mg/kg (oral, rat) |
| Related compounds | |
|
Other anions
|
Calcium chloride |
|
Other cations
|
Sodium hypochlorite |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Calcium hypochlorite is an inorganic compound with formula Ca(ClO)2. As a mixture with lime and calcium chloride, it is marketed as chlorine powder or bleach powder for water treatment and as a bleaching agent. This compound is relatively stable and has greater available chlorine than sodium hypochlorite (liquid bleach). It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. It is not highly soluble in water and is more preferably used in soft to medium-hard water. It has two forms: dry and hydrated.
Calcium hypochlorite is commonly used to sanitize public swimming pools and disinfect drinking water. Generally the commercial substance is sold with a purity of a 68% (with other additives and contaminants varying based upon the product's intended purpose). For instance as a swimming pool chemical it is often mixed with cyanuric acid stabilizers and anti-scaling agents (in order to reduce the loss of chlorine from ultraviolet radiation and to prevent calcium hardening). Calcium hypochlorite is also used in kitchens to disinfect surfaces and equipment. Other common uses include bathroom cleansers, household disinfectant sprays, algaecides, herbicides, and laundry detergents.
Calcium hypochlorite is a general oxidizing agent and therefore finds some use in organic chemistry. For instance the compound is used to cleave glycols, α-hydroxy carboxylic acids and keto acids to yield fragmented aldehydes or carboxylic acids. Calcium hypochlorite can also be used in the haloform reaction to manufacture chloroform.
...
Wikipedia

