CTP synthase
| CTP synthetase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||
| Identifiers | |||||||||
| EC number | 6.3.4.2 | ||||||||
| CAS number | 9023-56-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
CTP synthetase is an enzyme involved in pyrimidine biosynthesis that interconverts UTP and CTP.
CTP (cytidine triphosphate) synthetase catalyzes the last committed step in pyrimidine nucleotide biosynthesis:
ATP + UTP + glutamine → ADP + Pi + CTP + glutamate
It is the rate-limiting enzyme for the synthesis of cytosine nucleotides from both the de novo and uridine salvage pathways.
The reaction proceeds by the ATP-dependent phosphorylation of UTP on the 4-oxygen atom, making the 4-carbon electrophilic and vulnerable to reaction with ammonia. The source of the amino group in CTP is glutamine, which is hydrolysed in a glutamine amidotransferase domain to produce ammonia. This is then channeled through the interior of the enzyme to the synthetase domain. Here, ammonia reacts with the intermediate 4-phosphoryl UTP.
Two isozymes with CTP synthase activity exist in humans, encoded by the following genes:
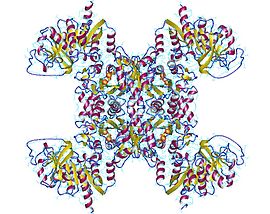
Active CTP synthase exists as a homotetrameric enzyme. At low enzyme concentrations and in the absence of ATP and UTP, CTP synthase exists as inactive monomer. As enzyme concentration increases, it polymerizes first to a dimer (such as the form shown to the left) and, in the presence of ATP and UTP, forms a tetramer.
The enzyme contains two major domains, responsible for the aminotransferase and synthase activity, respectively. The amidotransferase domains are located away from the tetramer interfaces and are not affected by the oligomeric state. The ATP-binding site and CTP-binding site in the synthase domain are located at the tetramer interface. It is for this reason that ATP and UTP are required for tetramerization.
CTP synthase is precisely regulated by the intracellular concentrations of CTP and UTP, and both hCTPS1 and hCTPS2 have been seen to be maximally active at physiological concentrations of ATP, GTP, and glutamine.
The activity of human CTPS1 isozyme has been demonstrated to be inhibited by phosphorylation. One major example of this is phosphorylation of the Ser-571 residue by glycogen synthase kinase 3 (GSK3) in response to low serum conditions. Additionally, Ser568 has been seen to be phosphorylated by casein kinase 1, inhibiting CTP synthase activity.
CTP is also subject to various forms of allosteric regulation. GTP acts as an allosteric activator that strongly promotes the hydrolysis of glutamine, but is also inhibiting to glutamine-dependent CTP formation at high concentrations. This acts to balance the relative amounts of purine and pyrimidine nucleotides. The reaction product CTP also serves as an allosteric inhibitor. The triphosphate binding site overlaps with that of UTP, but the nucleoside moiety of CTP binds in an alternative pocket opposite the binding site for UTP.
...
Wikipedia
