Boniva (drug)
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0.6% |
| Protein binding | 90.9 to 99.5% (concentration-dependent) |
| Metabolism | Nil |
| Biological half-life | 10 to 60 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.214.537 |
| Chemical and physical data | |
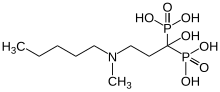
| Formula | C9H23NO7P2 |
| Molar mass | 319.229 g/mol |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Ibandronic acid (INN) or ibandronate sodium (USAN) is a potent bisphosphonate drug developed by Hoffman La Roche and used in the prevention and treatment of osteoporosis and metastasis-associated skeletal fractures in people with cancer. It may also be used to treat hypercalcemia (elevated blood calcium levels).
Ibandronate is indicated for the treatment and prevention of osteoporosis in post-menopausal women. In May 2003, the U.S. Food and Drug Administration (FDA) approved Ibandronate as a daily treatment for post-menopausal osteoporosis. The basis for this approval was a three-year, randomized, double-blind, placebo-controlled trial women with post-menopausal osteoporosis. Every participant also received daily oral doses of calcium and 400IUs [international units] of vitamin D. At the study's conclusion, both doses significantly reduced the occurrence risk of new vertebral fractures by 50–52 percent when compared to the effects of the placebo drug.
Ibandronate is efficacious for the prevention of metastasis-related bone fractures in multiple myeloma, breast cancer, and certain other cancers.
In 2008, the U.S Food and Drug Administration (FDA) issued a communication warning of the possibility of severe and sometimes incapacitating bone, joint or muscle pain. A study conducted by the American Society of Bone and Mineral Research concluded that long-term use of bisphosphonates, including Boniva, may increase the risk of a rare but serious fracture of the femur.
...
Wikipedia
