Bis(benzonitrile)palladium dichloride
 |
|
| Names | |
|---|---|
| Other names
palladium dichloride sis(benzonitrile), bis(benzonitrile)dichloropalladium
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| EC Number | 238-085-3 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C14H10Cl2N2Pd | |
| Molar mass | 383.57 g·mol−1 |
| Appearance | yellow-brown |
| Melting point | 129–130 °C (264–266 °F; 402–403 K) |
| Hazards | |
| GHS pictograms |
 
|
| GHS signal word | Danger |
| H301, H311, H330 | |
| P260, P261, P264, P270, P271, P273, P280, P284, P301+310, P301+312, P302+352, P304+312, P304+340, P305+351+338, P310, P311, P312, P320, P321, P322, P330, P332+313, P337+313, P361, P362 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
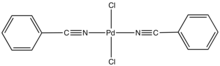
Bis(benzonitrile)palladium dichloride is the coordination complex with the formula PdCl2(NCC6H5)2. It is the adduct of two benzonitrile (PhCN) ligands with palladium(II) chloride. It is a yellow-brown solid that is soluble in organic solvents. The compound is a reagent and a catalyst for reactions that require soluble Pd(II). A closely related compound is bis(acetonitrile)palladium dichloride.
The complex is prepared by dissolving PdCl2 in warm benzonitrile. The PhCN ligands are labile, and the complex reverts to PdCl2 in noncoordinating solvents. According to X-ray crystallography, the two PhCN ligands are mutually trans.
...
Wikipedia
