Bestatin
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
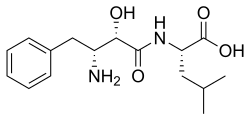
(2S)-2-[[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic acid
|
|
| Other names
Bestatin; N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine
|
|
| Identifiers | |
|
58970-76-6 65391-42-6 (HCl) |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL29292 |
| ChemSpider | 65145 |
| ECHA InfoCard | 100.055.917 |
| PubChem | 72172 |
| UNII |
I0J33N5627 |
|
|
|
|
| Properties | |
| C16H24N2O4 | |
| Molar mass | 308.38 g·mol−1 |
| Melting point | 245 °C (473 °F; 518 K) (decomposes) |
| Hazards | |
| S-phrases | S22 S24/25 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B),leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities),alanyl aminopeptidase (aminopeptidase M/N),leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of , vasopressin, enkephalins, and various other peptides and compounds.
...
Wikipedia
