Antifreeze protein
| Insect antifreeze protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
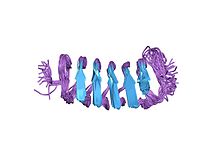
Structure of the Tenebrio molitor beta-helical antifreeze protein
|
|||||||||
| Identifiers | |||||||||
| Symbol | AFP | ||||||||
| Pfam | PF02420 | ||||||||
| InterPro | IPR003460 | ||||||||
| SCOP | 1ezg | ||||||||
| SUPERFAMILY | 1ezg | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| Choristoneura fumiferana antifreeze protein (CfAFP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|

Structure of Choristoneura fumiferana (spruce budworm) beta-helical antifreeze protein
|
|||||||||
| Identifiers | |||||||||
| Symbol | CfAFP | ||||||||
| Pfam | PF05264 | ||||||||
| InterPro | IPR007928 | ||||||||
| SCOP | 1m8n | ||||||||
| SUPERFAMILY | 1m8n | ||||||||
| OPM superfamily | 392 | ||||||||
| OPM protein | 1l0s | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
Antifreeze proteins (AFPs) or ice structuring proteins (ISPs) refer to a class of polypeptides produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind to small ice crystals to inhibit growth and recrystallization of ice that would otherwise be fatal. There is also increasing evidence that AFPs interact with mammalian cell membranes to protect them from cold damage. This work suggests the involvement of AFPs in cold acclimatization.
Unlike the widely used automotive antifreeze, ethylene glycol, AFPs do not lower freezing point in proportion to concentration. Rather, they work in a noncolligative manner. This phenomenon allows them to act as an antifreeze at concentrations 1/300th to 1/500th of those of other dissolved solutes. Their low concentration minimizes their effect on osmotic pressure. The unusual properties of AFPs are attributed to their selective affinity for specific crystalline ice forms and the resulting blockade of the ice-nucleation process.
AFPs create a difference between the melting point and freezing point known as thermal hysteresis. The addition of AFPs at the interface between solid ice and liquid water inhibits the thermodynamically favored growth of the ice crystal. Ice growth is kinetically inhibited by the AFPs covering the water-accessible surfaces of ice.
Thermal hysteresis is easily measured in the lab with a nanolitre osmometer. Organisms differ in their values of thermal hysteresis. The maximum level of thermal hysteresis shown by fish AFP is approximately -1.5 °C (29.3 °F). However, insect antifreeze proteins are 10–30 times more active than fish proteins. This difference probably reflects the lower temperatures encountered by insects on land. In contrast, aquatic organisms are exposed only to -1 to -2 °C below freezing. During the extreme winter months, the spruce budworm resists freezing at temperatures approaching -30 °C. The Alaskan beetle Upis ceramboides can survive in a temperature of -60 °C by using antifreeze agents that are not proteins.
...
Wikipedia
