Aluminum trichloride
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
aluminium chloride
|
|
| Other names
aluminium(III) chloride
aluminum trichloride |
|
| Identifiers | |
|
|
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.371 |
| 1876 | |
|
PubChem CID
|
|
| RTECS number | BD0530000 |
| UNII | |
|
|
|
|
| Properties | |
| AlCl3 | |
| Molar mass | 133.341 g/mol (anhydrous) 241.432 g/mol (hexahydrate) |
| Appearance | white or pale yellow solid, hygroscopic |
| Density | 2.48 g/cm3 (anhydrous) 2.398 g/cm3 (hexahydrate) |
| Melting point | 192.6 °C (378.7 °F; 465.8 K) (anhydrous) 100 °C (212 °F; 373 K) (hexahydrate, dec.) |
| Boiling point | 180 °C (356 °F; 453 K) (sublimates) |
| 439 g/l (0 °C) 449 g/l (10 °C) 458 g/l (20 °C) 466 g/l (30 °C) 473 g/l (40 °C) 481 g/l (60 °C) 486 g/l (80 °C) 490 g/l (100 °C) |
|
| Solubility | soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride slightly soluble in benzene |
| Vapor pressure | 133.3 Pa (99 °C) 13.3 kPa (151 °C) |
| Viscosity | 0.35 cP (197 °C) 0.26 cP (237 °C) |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
|
a = 0.591 nm, b = 0.591 nm, c = 1.752 nm
|
|
|
Lattice volume (V)
|
0.52996 nm3 |
|
Formula units (Z)
|
6 |
| Octahedral (solid) Tetrahedral (liquid) |
|
| Trigonal planar (monomeric vapour) |
|
| Thermochemistry | |
| 91 J/mol·K | |
|
Std molar
entropy (S |
111 J/mol·K |
|
Std enthalpy of
formation (ΔfH |
−704.2 kJ/mol |
|
Gibbs free energy (ΔfG˚)
|
-628.6 kJ/mol |
| Pharmacology | |
| D10AX01 (WHO) | |
| Hazards | |
| Safety data sheet | See: data page |
| GHS pictograms |  |
| GHS signal word | Danger |
| H314 | |
| P280, P310, P305+351+338 | |
|
EU classification (DSD)
|
|
| R-phrases | R34 |
| S-phrases | (S1/2), S7/8, S28, S45 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
anhydrous: 380 mg/kg, rat (oral) hexahydrate: 3311 mg/kg, rat (oral) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
none |
|
REL (Recommended)
|
2 mg/m3 |
|
IDLH (Immediate danger)
|
N.D. |
| Related compounds | |
|
Other anions
|
Aluminium fluoride Aluminium bromide Aluminium iodide |
|
Other cations
|
Boron trichloride Gallium trichloride Indium(III) chloride Magnesium chloride |
|
Related Lewis acids
|
Iron(III) chloride Boron trifluoride |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Aluminium chloride (AlCl3) is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron(III) chloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of chemical industry. The compound is often cited as a Lewis acid. It is an example of an inorganic compound that "cracks" at mild temperature, reversibly changing from a polymer to a monomer.
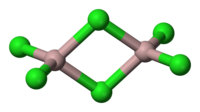
AlCl3 adopts three different structures, depending on the temperature and the state (solid, liquid, gas). Solid AlCl3 is a sheet-like layered cubic close packed layers. In this framework, the Al centres exhibit octahedral coordination geometry. In the melt, aluminium trichloride exists as the dimer Al2Cl6, with tetracoordinate aluminium. This change in structure is related to the lower density of the liquid phase (1.78 g/cm3) vs solid aluminium trichloride (2.48 g/cm3). Al2Cl6 dimers are also found in the vapour phase. At higher temperatures, the Al2Cl6 dimers dissociate into trigonal planar AlCl3, which is structurally analogous to BF3. The melt conducts electricity poorly, unlike more ionic halides such as sodium chloride.
The hexahydrate consists of octahedral [Al(H2O)6]3+ centers and chloride counterions. Hydrogen bonds link the cation and anions. The hydrated form of aluminium chloride has an octahedral molecular geometry, with the central aluminum ion surrounded by six water ligand molecules. This means that the hydrated form cannot act as a Lewis acid since it cannot accept electron pairs, and thus this cannot be used as a catalyst in Friedel-Crafts alkylation of aromatic compounds.
...
Wikipedia

