Alpha lipoic acid
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
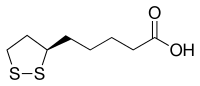
(R)-5-(1,2-Dithiolan-3-yl)pentanoic acid
|
|
| Other names
α-Lipoic acid; Alpha lipoic acid; Thioctic acid; 6,8-Dithiooctanoic acid
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.486 |
| KEGG | |
| MeSH | Lipoic+acid |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C8H14O2S2 | |
| Molar mass | 206.32 g·mol−1 |
| Appearance | Yellow needle-like crystals |
| Melting point | 46–48 °C (115–118 °F; 319–321 K) |
| Soluble as sodium salt | |
| Solubility in ethanol | Soluble |
| Pharmacology | |
| A16AX01 (WHO) | |
| Pharmacokinetics: | |
| 30% (oral) | |
| Related compounds | |
|
Related compounds
|
Lipoamide Asparagusic acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lipoic acid (LA), also known as α-lipoic acid and alpha lipoic acid (ALA) and thioctic acid is an organosulfur compound derived from octanoic acid. ALA is made in animals normally, and is essential for aerobic metabolism. It is also manufactured and is available as a dietary supplement in some countries where it is marketed as an antioxidant, and is available as a pharmaceutical drug in other countries.
Lipoic acid (LA), also known as α-lipoic acid and alpha lipoic acid (ALA) and thioctic acid is an organosulfur compound derived from octanoic acid. LA contains two sulfur atoms (at C6 and C8) connected by a disulfide bond and is thus considered to be oxidized although either sulfur atom can exist in higher oxidation states.
The carbon atom at C6 is chiral and the molecule exists as two enantiomers (R)-(+)-lipoic acid (RLA) and (S)-(-)-lipoic acid (SLA) and as a racemic mixture (R/S)-lipoic acid (R/S-LA).
LA appears physically as a yellow solid and structurally contains a terminal carboxylic acid and a terminal dithiolane ring.
For use in dietary supplement materials and compounding pharmacies, the USP has established an official monograph for R/S-LA.
"Lipoate" is the conjugate base of lipoic acid, and the most prevalent form of LA under physiologic conditions. Most endogenously produced RLA is not "free" because octanoic acid, the precursor to RLA, is bound to the enzyme complexes prior to enzymatic insertion of the sulfur atoms. As a cofactor, RLA is covalently attached by an amide bond to a terminal lysine residue of the enzyme's lipoyl domains. One of the most studied roles of RLA is as a cofactor of the pyruvate dehydrogenase complex (PDC or PDHC), though it is a cofactor in other enzymatic systems as well (described below).
...
Wikipedia
