Allicin
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
S-Prop-2-en-1-yl prop-2-ene-1-sulfinothioate
|
|
| Other names
2-Propene-1-sulfinothioic acid S-2-propenyl ester
3-[(Prop-2-ene-1-sulfinyl)sulfanyl]prop-1-ene S-Allyl prop-2-ene-1-sulfinothioate |
|
| Identifiers | |
|
539-86-6 |
|
| 3D model (Jmol) |
Interactive image Interactive image |
| 1752823 | |
| ChEBI |
CHEBI:28411 |
| ChEMBL |
ChEMBL359965 |
| ChemSpider |
58548 |
| ECHA InfoCard | 100.007.935 |
| EC Number | 208-727-7 |
| 2419 | |
| KEGG |
C07600 |
| MeSH | Allicin |
| PubChem | 65036 |
| UNII |
3C39BY17Y6 |
|
|
|
|
| Properties | |
| C6H10OS2 | |
| Molar mass | 162.26 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.112 g cm−3 |
| Melting point | <25 °C |
| Boiling point | decomposes |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. The allicin generated is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is part of a defense mechanism against attacks by pests on the garlic plant.
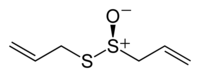
Allicin features the thiosulfinate functional group, R-S(O)-S-R. The compound is not present in garlic unless tissue damage occurs, and is formed by the action of the enzyme alliinase on alliin. Allicin is chiral but occurs naturally only as a racemate. The racemic form can also be generated by oxidation of diallyl disulfide:
Alliinase is irreversibly deactivated below pH 3; as such, allicin is generally not produced in the body from the consumption of fresh or powdered garlic. Furthermore, allicin can be unstable, breaking down within 16 hours at 23 °C.
Allicin is an oily, slightly yellow liquid that gives garlic its unique odor. It is a thioester of sulfenic acid and is also known as allyl thiosulfinate. Its biological activity can be attributed to both its antioxidant activity and its reaction with thiol-containing proteins.
In the biosynthesis of allicin (thio-2-propene-1-sulfinic acid S-allyl ester), cysteine is first converted into alliin (+ S-allyl-L-cysteine sulfoxide). The enzyme alliinase, which contains pyridoxal phosphate (PLP), cleaves alliin, generating allysulfenic acid, pyruvate, and ammonium. At room temperature allysulfenic acid is unstable and highly reactive, which cause two molecules of it to spontaneously combine in a dehydration reaction to form allicin.
...
Wikipedia
