Adeno-associated virus
| Adeno-associated virus | |
|---|---|
 |
|
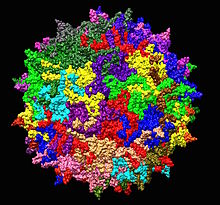
| Adeno-associated virus serotype 2 structure from 1LP3. One fivefold axis shown center. | |
| Virus classification | |
| Group: | Group II (ssDNA) |
| Order: | Unassigned |
| Family: | Parvoviridae |
| Subfamily: | Parvovirinae |
| Genus: | Dependoparvovirus |
| Species | |
|
|
Adeno-associated virus (AAV) is a small virus which infects humans and some other primate species. AAV is not currently known to cause disease. The virus causes a very mild immune response, lending further support to its apparent lack of pathogenicity. Gene therapy vectors using AAV can infect both dividing and quiescent cells and persist in an extrachromosomal state without integrating into the genome of the host cell, although in the native virus some integration of virally carried genes into the host genome does occur. These features make AAV a very attractive candidate for creating viral vectors for gene therapy, and for the creation of isogenic human disease models. Recent human clinical trials using AAV for gene therapy in the retina have shown promise.
AAV belongs to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. The virus is a small (20 nm) replication-defective, nonenveloped virus.
The adeno-associated virus (AAV), previously thought to be a contaminant in adenovirus preparations, was first identified as a dependovirus in the 1960s in the laboratories of Bob Atchison at Pittsburgh and Wallace Rowe at NIH. Serological studies in humans subsequently indicated that, despite being present in people infected by helper viruses such as adenovirus or herpes virus, AAV itself did not cause any disease.
Wild-type AAV has attracted considerable interest from gene therapy researchers due to a number of features. Chief amongst these is the virus's apparent lack of pathogenicity. It can also infect non-dividing cells and has the ability to stably integrate into the host cell genome at a specific site (designated AAVS1) in the human chromosome 19. This feature makes it somewhat more predictable than retroviruses, which present the threat of a random insertion and of mutagenesis, which is sometimes followed by development of a cancer. The AAV genome integrates most frequently into the site mentioned, while random incorporations into the genome take place with a negligible frequency. Development of AAVs as gene therapy vectors, however, has eliminated this integrative capacity by removal of the rep and cap from the DNA of the vector. The desired gene together with a promoter to drive transcription of the gene is inserted between the inverted terminal repeats (ITR) that aid in concatemer formation in the nucleus after the single-stranded vector DNA is converted by host cell DNA polymerase complexes into double-stranded DNA. AAV-based gene therapy vectors form episomal concatemers in the host cell nucleus. In non-dividing cells, these concatemers remain intact for the life of the host cell. In dividing cells, AAV DNA is lost through cell division, since the episomal DNA is not replicated along with the host cell DNA. Random integration of AAV DNA into the host genome is detectable but occurs at very low frequency. AAVs also present very low immunogenicity, seemingly restricted to generation of neutralizing antibodies, while they induce no clearly defined cytotoxic response. This feature, along with the ability to infect quiescent cells present their dominance over adenoviruses as vectors for human gene therapy.
...
Wikipedia
