Acid regeneration
Hydrochloric acid regeneration or HCl regeneration refers to a chemical process for the reclamation of bound and unbound HCl from metal chloride solutions such as hydrochloric acid.
The commercially most relevant field of application for HCl regeneration processes is the recovery of HCl from waste pickle liquors from carbon-steel pickling lines. Other applications include the production of metal oxides such as, but not limited, to Al2O3 and MgO, as well as rare-earth oxides, by pyrohydrolysis of aqueous metal chloride or rare-earth chloride solutions.
A number of different process routes are available. The most widely used is based on pyrohydrolysis and adiabatic absorption of hydrogen chloride in water, a process invented in the 1960s. However tightening environmental standards and stringent air permit policies render it increasingly difficult to establish new pyrohydrolysis-based acid regeneration plants.
The following processes for the regeneration of HCl from spent pickle liquors have been adopted by the ferrous metals processing industry:
Hydrothermal hydrolysis of hydrochloric SPL from carbon-steel pickling lines is a hydrometallurgical reaction, which takes place according to the following chemical formulae:
12 FeCl2 + 3 O2 → 8 FeCl3 + 2 Fe2O3
2 FeCl3 + 3 H2O → 6 HCl + Fe2O3
Today hydrothermal hydrolysis, which operates at very low temperatures, consumes only a fraction of the energy other processes demand and produces virtually no emissions, is considered the most effective way to regenerate any given quantity of spent pickle liquor.
Known implementations of the hydrothermal HCl regeneration processes include the PORI process (1974 for J&L Steel, dismantled) and the optimized SMS Demag wet process (2008 for ThyssenKrupp Steel, under construction).
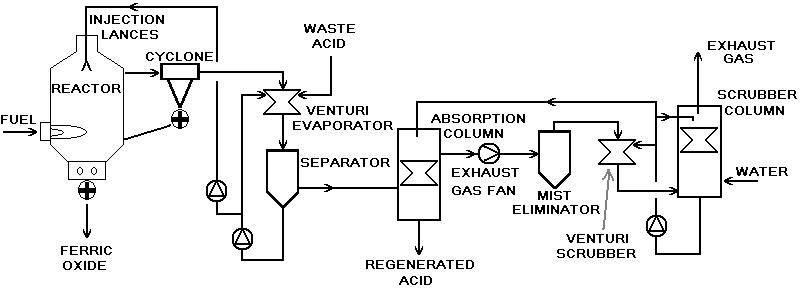
Pyrohydrolysis of hydrochloric spent pickle liquor from carbon steel pickling lines is a hydrometallurgical reaction which takes place according to the following chemical formulae:
4 FeCl2 + 4 H2O + O2 = 8 HCl + 2 Fe2O3
2 FeCl3 + 3 H2O = 6 HCl + Fe2O3
The process is an inversion of the chemical descaling (pickling) process.
...
Wikipedia