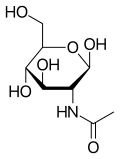
Acetylglucosamine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
β-D-(Acetylamino)-2-deoxy-glucopyranose
|
|
| Other names
N-Acetyl-D-glucosamine
GlcNAc NAG |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.517 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C8H15NO6 | |
| Molar mass | 221.21 |
| Melting point | 211 |
| Hazards | |
| S-phrases (outdated) | S24/25 |
| Related compounds | |
|
Related Monosaccharides
|
N-Acetylgalactosamine |
|
Related compounds
|
Glucosamine Glucose |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
N-Acetylglucosamine (N-acetyl-D-glucosamine, or GlcNAc, or NAG) is a monosaccharide and a derivative of glucose. It is an amide between glucosamine and acetic acid. It has a molecular formula of C8H15NO6, a molar mass of 221.21 g/mol, and it is significant in several biological systems.
It is part of a biopolymer in the bacterial cell wall, which is built from alternating units of GlcNAc and N-acetylmuramic acid (MurNAc), cross-linked with oligopeptides at the lactic acid residue of MurNAc. This layered structure is called peptidoglycan (formerly called murein).
GlcNAc is the monomeric unit of the polymer chitin, which forms the outer coverings of insects and crustaceans. It is the main component of the radulas of mollusks, the beaks of cephalopods, and a major component of the cell walls of most fungi.
Polymerized with glucuronic acid, it forms hyaluronan.
GlcNAc has been reported to be an inhibitor of elastase release from human polymorphonuclear leukocytes (range 8–17% inhibition), however this is much weaker than the inhibition seen with N-acetylgalactosamine (range 92–100%).
It has been proposed as a treatment for autoimmune diseases, and recent tests have claimed some success.
...
Wikipedia
