Acetylcysteine
 |
|
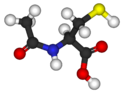 |
|
| Clinical data | |
|---|---|
| Pronunciation | /əˌsɛtəlˈsɪstiːn/ |
| Trade names | Acetadote, Fluimucil, Mucomyst, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
By mouth, injection, inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10% (Oral) |
| Protein binding | 50 to 83% |
| Metabolism | Liver |
| Biological half-life | 5.6 hours |
| Excretion | Renal (30%), faecal (3%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.009.545 |
| Chemical and physical data | |
| Formula | C5H9NO3S |
| Molar mass | 163.195 |
| 3D model (Jmol) | |
| Specific rotation | +5° (c = 3% in water) |
| Melting point | 109 to 110 °C (228 to 230 °F) |
|
|
|
|
Acetylcysteine, also known as N-acetylcysteine or N-acetyl-L-cysteine (NAC), is a medication used to treat paracetamol (acetaminophen) overdose and to loosen thick mucus such as in cystic fibrosis or chronic obstructive pulmonary disease. It can be taken intravenously, by mouth, or inhaled as a mist.
Common side effects include nausea and vomiting when taken by mouth. The skin may occasionally become red and itchy with either form. A non immune type of anaphylaxis may also occur. It appears to be safe in pregnancy. It works by increasing glutathione levels and binding with the toxic breakdown products of paracetamol.
Acetylcysteine was initially patented in 1960 and licensed for use in 1968. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. It is available as a generic medication and is not very expensive.
Intravenous and oral formulations of acetylcysteine are available for the treatment of paracetamol (acetaminophen) overdose. When paracetamol is taken in large quantities, a minor metabolite called N-acetyl-p-benzoquinone imine (NAPQI) accumulates within the body. It is normally conjugated by glutathione, but when taken in excess, the body's glutathione reserves are not sufficient to inactivate the toxic NAPQI. This metabolite is then free to react with key hepatic enzymes, thereby damaging . This may lead to severe liver damage and even death by acute liver failure.
...
Wikipedia
