Acetolactate synthase
| acetolactate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
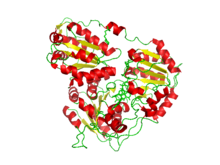
Crystal structure of Arabidopsis thaliana acetohydroxyacid synthase complexed with a sulfonylurea herbicide, metsulfuron-methyl.
|
|||||||||
| Identifiers | |||||||||
| EC number | 2.2.1.6 | ||||||||
| CAS number | 9027-45-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
| ilvB (bacterial acetolactate synthase)-like | |
|---|---|
| Identifiers | |
| Symbol | ILVBL |
| Entrez | 10994 |
| HUGO | 6041 |
| OMIM | 605770 |
| RefSeq | NM_176826 |
| UniProt | Q99651 |
| Other data | |
| EC number | 2.2.1.6 |
| Locus | Chr. 19 p13.1 |
The acetolactate synthase (ALS) enzyme (also known as acetohydroxy acid synthase, or AHAS) is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids (valine, leucine, and isoleucine).
It is a human protein of yet unknown function, sharing some sequence similarity with bacterial ALS, and is encoded by the ILVBL gene.
Human ILVBL gene has 17 exons resides on chromosome 19 at q13.1.
Acetolactase is a protein consisting of 590 residues. These residues are classified into three separate subunits. The units are d1yhya1, d1yhya2, and d1yhya3. This is classified by the SCOP domain assignments.
The structure of acetolactate synthase that was used for the picture on this page was determined using X-ray diffraction at 2.70 angstroms. X-ray diffraction uses X-rays at specified wavelengths to produce patterns, as the X–ray is scattered in certain ways that give an idea to the structure of the molecule being analyzed.
There are five specific ligands that interact with this protein. The five are listed below.
This certain protein is an enzyme involving catalytic activity, to be more specific, a part of the biosynthesis of various amino acids. This enzyme has the Enzyme Commission Code is 2.2.1.6, which means that the enzyme is a transketolase or a transaldolase, which is classified under the transferases that transfer aldehyde or ketone residues. In this case, acetolactase synthase is a transketolase, which moves back and forth, having both catabolic and anabolic forms. These act on a ketone (pyruvate) and can go back and forth in the metabolic chain. These are found in humans, animals, plants, and bacteria. In plants, they are located in the chloroplasts in order to help with the metabolic processes. In several experiments, it has been shown that mutated strains of Escherichia coli K-12 without the enzyme were not able to grow in the presence of only acetate or oleate as the only carbon sources.
Acetolactate synthesis, also known as acetohydroxy acid synthase, is an enzyme specifically involved in the chemical reaction involving the conversion of two pyruvate molecules to an acetolactate molecule and carbon dioxide. The reaction uses thiamine pyrophosphate in order to link the two pyruvate molecules. The resulting product of this reaction, acetolactate, eventually becomes valine, leucine, and isoleucine. All three of these amino acids are essential amino acids and cannot be synthesized in the organism.
...
Wikipedia
