4-Nitroaniline
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
4-Nitroaniline
|
|||
|
Systematic IUPAC name
4-Nitrobenzenamine
|
|||
| Other names
p-Nitroaniline
1-Amino-4-nitrobenzene p-Nitrophenylamine |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.555 | ||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C6H6N2O2 | |||
| Molar mass | 138.12 g/mol | ||
| Appearance | yellow or brown powder | ||
| Odor | faint, ammonia-like | ||
| Density | 1.437 g/ml, solid | ||
| Melting point | 146 to 149 °C (295 to 300 °F; 419 to 422 K) (lit.) | ||
| Boiling point | 332 °C (630 °F; 605 K) | ||
| 0.8 mg/ml at 18.5 °C (IPCS) | |||
| Vapor pressure | 0.00002 mmHg (20°C) | ||
| -66.43·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Toxic | ||
| Safety data sheet | JT Baker | ||
|
EU classification (DSD)
|
|
||
| R-phrases | R23/24/25 R33 R52/53 | ||
| S-phrases | S28 S36/37 S45 S61 | ||
| NFPA 704 | |||
| Flash point | 199 °C (390 °F; 472 K) | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
3249 mg/kg (rat, oral) 750 mg/kg (rat, oral) 450 mg/kg (guinea pig, oral) 810 mg/kg (mouse, oral) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 6 mg/m3 (1 ppm) [skin] | ||
|
REL (Recommended)
|
TWA 3 mg/m3 [skin] | ||
|
IDLH (Immediate danger)
|
300 mg/m3 | ||
| Related compounds | |||
|
Related compounds
|
2-Nitroaniline, 3-Nitroaniline | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. It is an organic chemical compound, consisting of a phenyl group attached to an amino group which is para to a nitro group. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals, and gasoline, in gum inhibitors, poultry medicines, and as a corrosion inhibitor.
It is produced industrially via the amination of 4-nitrochlorobenzene:
Below is a laboratory synthesis of 4-nitroaniline from aniline. The key step in this reaction sequence is an electrophilic aromatic substitution to install the nitro group para to the amino group. After this reaction, a separation must be performed to remove 2-nitroaniline, which is also formed in a small amount during the reaction.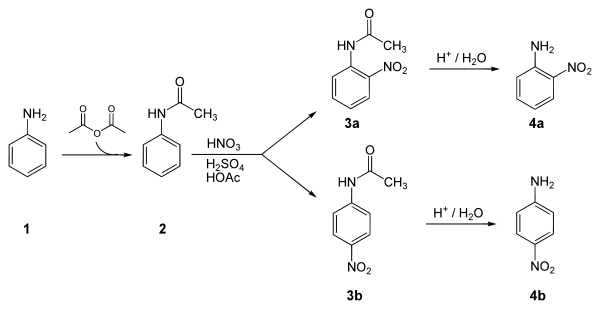
4-Nitroaniline is mainly consumed industrially as a precursor to p-phenylenediamine, an important dye component. The reduction is effected using iron metal and by catalytic hydrogenation.
It is a starting material for the synthesis of Para Red, the first azo dye:
When heated, it polymerizes explosively into a rigid foam.
Nitroaniline is a used for determining Kamlet-Taft solvent parameters. The position of its UV-visual peak changes with the balance of hydrogen bonding acceptors and donors in the solvent.
...
Wikipedia



