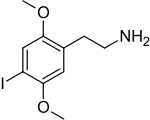
2C-I
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2-(4-Iodo-2,5-dimethoxyphenyl)ethan-1-amine
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.217.507 | ||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C10H14INO2 | |||
| Molar mass | 307.13 g·mol−1 | ||
| Melting point | 246 °C (475 °F; 519 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2C-I is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and described in his 1991 book PiHKAL: A Chemical Love Story. The drug is used recreationally for its psychedelic and entactogenic effects and is sometimes confused for the analog 25I-NBOMe, nicknamed "Smiles," in the media.
In the early 2000s, 2C-I was sold in Dutch smart shops after the drug 2C-B was banned.
According to the US Drug Enforcement Administration, 2C-I is taken orally or snorted in a powder form.
In December 2003, the European Council issued a binding order compelling all EU member states to ban 2C-I within three months.
As of October 31st, 2016, 2C-I is a controlled substance (Schedule III) in Canada.
2C-I is a schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015). A schedule 9 drug is outlined in the Poisons Act 1964 as "Substances which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of the CEO."
Sveriges riksdag added 2C-I to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Mar 16, 2004, published by Medical Products Agency in their regulation LVFS 2004:3 listed as 4-jodo-2,5-dimetoxifenetylamin (2C-I).
In the United Kingdom, 2C-I is controlled as a Class A substance.
...
Wikipedia


