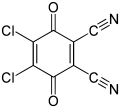
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
4,5-Dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile
|
|||
Other names
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| Abbreviations | DDQ | ||
| ChemSpider | |||
| ECHA InfoCard | 100.001.402 | ||
| EC Number | 201-542-2 | ||
|
PubChem CID
|
|||
| RTECS number | GU4825000 | ||
|
|||
|
|||
| Properties | |||
| C8Cl2N2O2 | |||
| Molar mass | 227.00 g·mol−1 | ||
| Appearance | yellow to orange powder | ||
| Density | 1.7g/cm3 | ||
| Melting point | 210 to 215 °C (410 to 419 °F; 483 to 488 K) (decomposes) | ||
| Boiling point | 301.8 °C (575.2 °F; 575.0 K) at 760mmHg | ||
| reacts | |||
| Hazards | |||
| R-phrases (outdated) | R25 R29 | ||
| S-phrases (outdated) | S22 S24/25 S37 S45 | ||
| Flash point | 136.3 °C (277.3 °F; 409.4 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.
Synthesis of DDQ involves cyanation of chloranil. Thiele and Günther first reported a 6-step preparation in 1906. The substance did not receive interest until its potential as a dehydrogenation agent was discovered. A single-step chlorination from 2,3-dicyanohydroquinone was reported in 1965.
The reagent removes pairs of H atoms from organic molecules. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene:
The resulting hydroquinone is poorly soluble in typical reaction solvents (dioxane, alkanes), which facilitates workup.
DDQ reacts with water with release of hydrogen cyanide (HCN), which is highly toxic. A low-temperature and weakly acidic environment increases the stability of DDQ.
...
Wikipedia