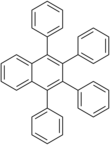
1,2,3,4-tetraphenylnaphthalene
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
1,2,3,4-Tetra(phenyl)naphthalene
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.151.838 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C34H24 | |||
| Molar mass | 432.55 g/mol | ||
| Melting point | 199 to 201 °C (390 to 394 °F; 472 to 474 K) | ||
| Hazards | |||
| R-phrases (outdated) | R36/37/38 | ||
| S-phrases (outdated) | S26 S36 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
1,2,3,4-Tetraphenylnaphthalene is a polycyclic aromatic hydrocarbon commonly prepared in the undergraduate teaching laboratory as an introduction to the Diels-Alder reaction, in this case between benzyne, which acts as the dienophile, (generated in situ) and tetraphenylcyclopentadienone, which acts as the diene. It has two crystalline forms, and therefore has two different melting points.
...
Wikipedia