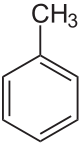
Toluene
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Toluene
|
|||
|
Systematic IUPAC name
Methylbenzene
|
|||
| Other names
Phenylmethane
Toluol Anisen |
|||
| Identifiers | |||
|
108-88-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| Abbreviations | PhMe MePh BnH |
||
| ChEBI |
CHEBI:17578 |
||
| ChEMBL |
ChEMBL9113 |
||
| ChemSpider |
1108 |
||
| DrugBank |
DB01900 |
||
| ECHA InfoCard | 100.003.297 | ||
| 5481 | |||
| KEGG |
C01455 |
||
| PubChem | 1140 | ||
| RTECS number | XS5250000 | ||
| UNII |
3FPU23BG52 |
||
|
|||
|
|||
| Properties | |||
| C7H8 | |||
| Molar mass | 92.14 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | sweet, pungent, benzene-like | ||
| Density | 0.87 g/mL (20 °C) | ||
| Melting point | −95 °C (−139 °F; 178 K) | ||
| Boiling point | 111 °C (232 °F; 384 K) | ||
| 0.52 g/L (20 °C) | |||
| Vapor pressure | 2.8 kPa (20°C) | ||
| -66.11·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.497 (20 °C) | ||
| Viscosity | 0.590 cP (20 °C) | ||
| Structure | |||
| 0.36 D | |||
| Hazards | |||
| Main hazards | highly flammable | ||
| Safety data sheet |
See: data page SIRI.org |
||
| R-phrases | R11, R38, R48/20, R63, R65, R67 | ||
| S-phrases | (S2), S36/37, S29, S46, S62 | ||
| NFPA 704 | |||
| Flash point | 6 °C (43 °F; 279 K) | ||
| Explosive limits | 1.1%-7.1% | ||
| 50 mL m−3, 190 mg m−3 | |||
| Lethal dose or concentration (LD, LC): | |||
|
LC50 (median concentration)
|
>26700 ppm (rat, 1 hr) 400 ppm (mouse, 24 hr) |
||
|
LCLo (lowest published)
|
55,000 ppm (rabbit, 40 min) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 200 ppm C 300 ppm 500 ppm (10-minute maximum peak) | ||
|
REL (Recommended)
|
TWA 100 ppm (375 mg/m3) ST 150 ppm (560 mg/m3) | ||
|
IDLH (Immediate danger)
|
500 ppm | ||
| Related compounds | |||
|
Related aromatic hydrocarbons
|
benzene xylene naphthalene |
||
|
Related compounds
|
methylcyclohexane | ||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Toluene /ˈtɒljuːiːn/, also known as toluol /ˈtɒljuːɒl/, is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a CH3 group attached to a phenyl group. As such, its IUPAC systematic name is methylbenzene. It is an aromatic hydrocarbon.
Toluene is widely used as an industrial and a solvent. In 2013, worldwide sales of toluene amounted to about 24.5 billion US-dollars.
As the solvent in some types of paint thinner, contact cement and model airplane glue, toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm.
The compound was first isolated in 1837 through a distillation of pine oil by a Polish chemist named Filip Walter, who named it rétinnaphte. In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree Myroxylon balsamum), which Deville recognized as similar to Walter's rétinnaphte and to benzene; hence he called the new hydrocarbon benzoène. In 1843, Jöns Jacob Berzelius recommended the name toluin. In 1850, French chemist Auguste Cahours isolated from a distillate of wood a hydrocarbon which he recognized as similar to Deville's benzoène and which Cahours named toluène.
...
Wikipedia