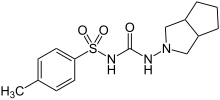
Gliclazide
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Diamicron, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 10.4 hours |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.040.221 |
| Chemical and physical data | |
| Formula | C15H21N3O3S |
| Molar mass | 323.412 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Gliclazide, sold under the brand name Diamicron among others, chemically a 1-(3-azabicyclo (3.3.0) oct-3-yl)-3-p-tolysulphonylurea is a second-generation sulfonylurea derivative, which is profoundly used for the treatment of type II diabetes mellitus and mostly available as oral tablets (30 and 80 mg strength) with the recommended dosage between 40 and 320 mg/day. It was also shown to protect human pancreatic beta-cells from hyperglycemia-induced apoptosis. It is used when dietary changes, exercise, and weight loss are not enough. It is taken by mouth.
Side effect may include low blood sugar, vomiting, abdominal pain, rash, and liver problems. Use by those with significant kidney problems, liver problems, or who are pregnancy is not recommended. Gliclazide is in the sulfonylurea family of medications. It works mostly by increasing the release of insulin.
Gliclazide was patented in 1966 and approved for medical use in 1972. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about 2.46 to 3.92 USD per month. In the United Kingdom a month of medication costs the NHS about 2.12 pounds. It is not available for sale in the United States.
Gliclazide is used for control of hyperglycemia in gliclazide-responsive diabetes mellitus of stable, mild, non-ketosis prone, type 2 diabetes. It is used when diabetes cannot be controlled by proper dietary management and exercise or when insulin therapy is not appropriate. National Kidney Foundation (2012 Update) claims that Gliclazide does not require dosage uptitration even in end stage kidney disease.
Hyperglycemic action may be caused by danazol, chlorpromazine, glucocorticoids, progestogens, or β-2 agonists. Its hypoglycemic action may be potentiated by phenylbutazone, alcohol, fluconazole, β-blockers, and possibly ACE inhibitors. It has been found that rifampin increases gliclazide metabolism in humans in vivo.
...
Wikipedia
