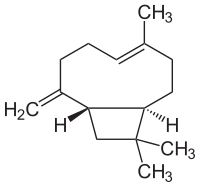
Caryophyllene
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
(1R,4E,9S)-4,11,11-Trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene
|
|
| Other names
(1R,9S,E)-4,11,11-Trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene
β-Caryophyllene trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene |
|
| Identifiers | |
|
87-44-5 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:10357 |
| ChEMBL |
ChEMBL445740 |
| ChemSpider |
4444848 |
| ECHA InfoCard | 100.001.588 |
| PubChem | 5281515 |
| UNII |
BHW853AU9H |
|
|
|
|
| Properties | |
| C15H24 | |
| Molar mass | 204.36 g·mol−1 |
| Density | 0.9052 g/cm3 (17 °C) |
| Boiling point | 254–257 °C (489–495 °F; 527–530 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Caryophyllene /ˌkærioʊfɪˈliːn/, or (−)-β-caryophyllene, is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa,rosemary, and hops. It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and α-humulene (obsolete name: α-caryophyllene), a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in an 8-membered ring, both rarities in nature.
The first total synthesis of caryophyllene in 1964 by E.J. Corey was considered one of the classic demonstrations of the possibilities of synthetic organic chemistry at the time.
Caryophyllene is one of the chemical compounds that contributes to the spiciness of black pepper.
14-Hydroxycaryophyllene oxide (C15H24O2) was isolated from the urine of rabbits treated with (-)-caryophyllene (C15H24). The x-ray crystal structure of 14-hydroxycaryophyllene (as its acetate derivative) has been reported.
The metabolism of caryophyllen progresses through (-)-caryophyllene oxide (C15H24O) since the latter compound also afforded 14-hydroxycaryophyllene (C15H24O) as a metabolite.
...
Wikipedia
